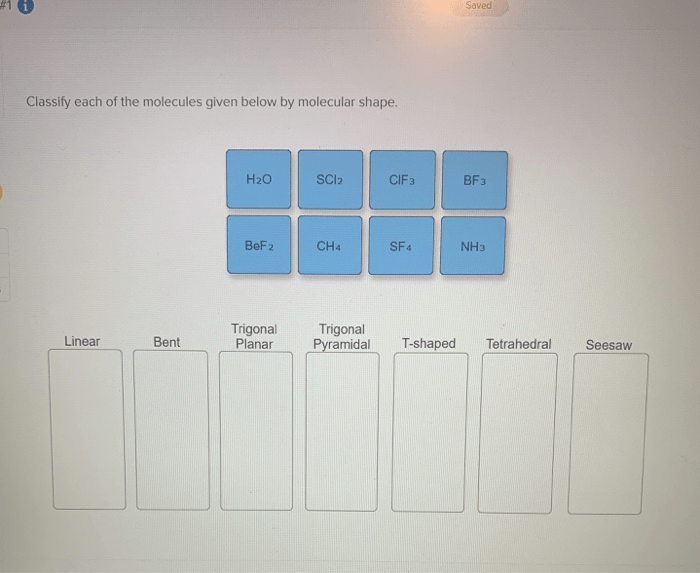
Classify each of the molecules given below by molecular shape. Molecular shape, the geometric arrangement of atoms within a molecule, plays a crucial role in determining its physical and chemical properties. This article explores the concept of molecular shape, its significance, and the methods used to determine it, providing insights into the fundamental nature of molecules.
Molecular shape influences various properties, including polarity, solubility, and reactivity. It finds applications in diverse fields, from chemistry and biology to materials science, enabling scientists to predict and understand molecular behavior.
Introduction: Classify Each Of The Molecules Given Below By Molecular Shape.
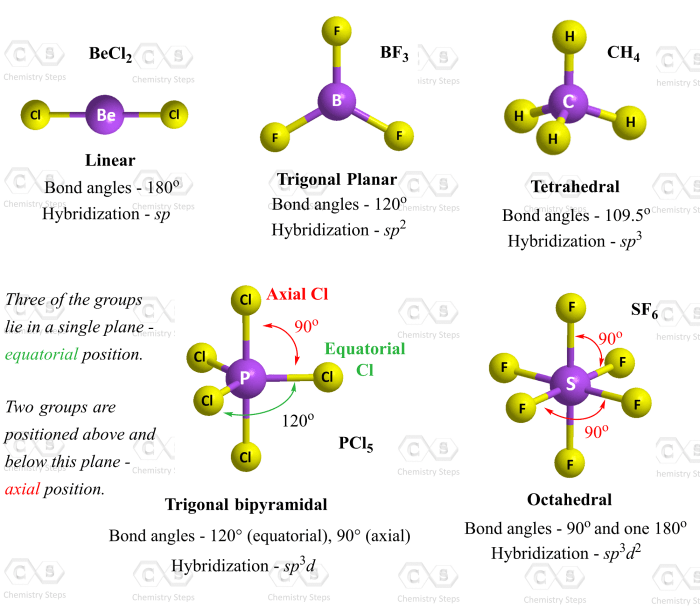
Molecular shape is the three-dimensional arrangement of atoms in a molecule. It is an important factor in determining the physical and chemical properties of a molecule.
The shape of a molecule can be determined by several methods, including VSEPR theory and X-ray crystallography. VSEPR theory is a simple method that can be used to predict the shape of a molecule based on the number of valence electrons in the molecule.
X-ray crystallography is a more accurate method that can be used to determine the exact shape of a molecule.
Types of Molecular Shapes
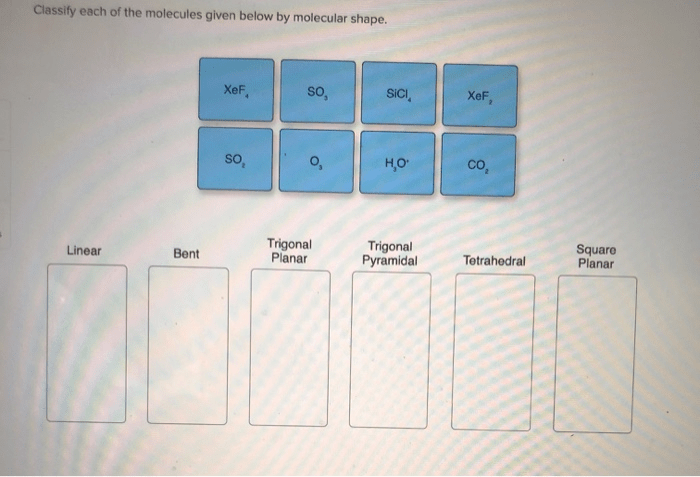
There are many different types of molecular shapes. The most common types of molecular shapes are:
- Linear
- Trigonal planar
- Tetrahedral
- Octahedral
Linear molecules have two atoms that are bonded together in a straight line. Trigonal planar molecules have three atoms that are bonded together in a flat triangle. Tetrahedral molecules have four atoms that are bonded together in a three-dimensional tetrahedron.
Octahedral molecules have six atoms that are bonded together in a three-dimensional octahedron.
Methods for Determining Molecular Shape
There are several different methods that can be used to determine the molecular shape of a molecule. The most common methods are:
- VSEPR theory
- X-ray crystallography
VSEPR theory is a simple method that can be used to predict the shape of a molecule based on the number of valence electrons in the molecule. X-ray crystallography is a more accurate method that can be used to determine the exact shape of a molecule.
Applications of Molecular Shape, Classify each of the molecules given below by molecular shape.
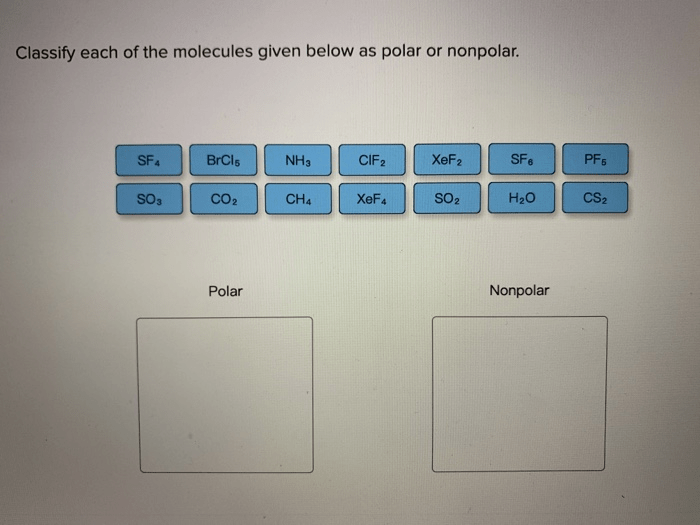
Molecular shape can be used to explain a variety of properties of molecules, such as polarity, solubility, and reactivity. Polarity is a measure of the uneven distribution of charge in a molecule. Soluble molecules are able to dissolve in water, while insoluble molecules are not.
Reactive molecules are able to react with other molecules, while unreactive molecules are not.
Molecular shape is also used in a variety of fields, such as chemistry, biology, and materials science. In chemistry, molecular shape is used to explain the properties of molecules and to design new molecules with specific properties. In biology, molecular shape is used to explain the structure and function of proteins and other biological molecules.
In materials science, molecular shape is used to design new materials with specific properties.
Key Questions Answered
What is the significance of molecular shape?
Molecular shape influences physical and chemical properties, such as polarity, solubility, and reactivity, providing insights into molecular behavior.
How is molecular shape determined?
Molecular shape can be determined using methods like VSEPR theory and X-ray crystallography, which predict the arrangement of atoms based on electron pair repulsion.
What are the different types of molecular shapes?
Common molecular shapes include linear, trigonal planar, tetrahedral, and octahedral, each with distinct geometric arrangements of atoms.