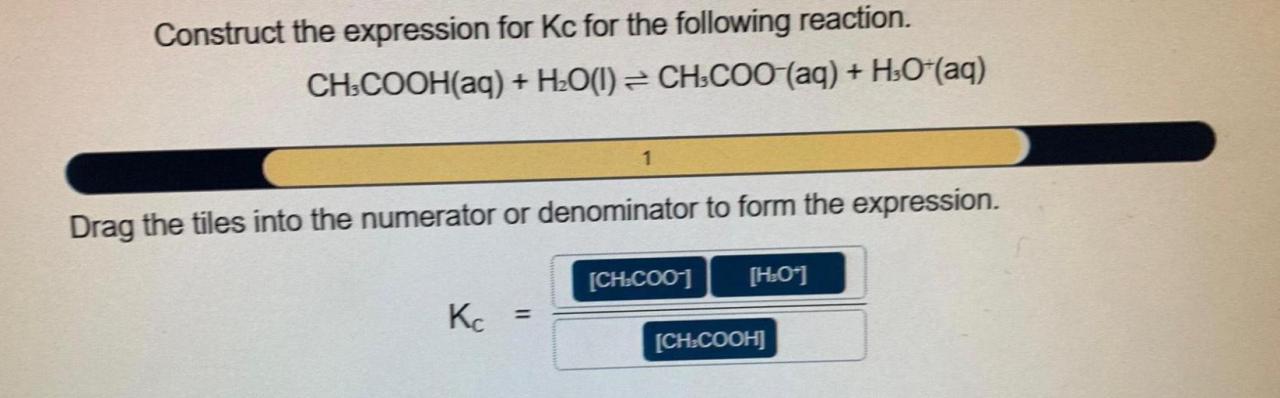
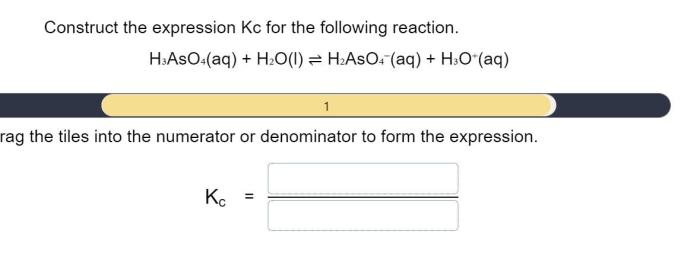
Construct the expression for kc for the following reaction. – Constructing the expression for Kc for a given reaction is a crucial step in understanding chemical equilibrium. This guide will provide a comprehensive overview of the concept of Kc, its significance, and the steps involved in constructing its expression.
The equilibrium constant, Kc, is a quantitative measure of the extent to which a chemical reaction proceeds towards completion. It provides valuable insights into the reaction’s spontaneity, direction, and the relative amounts of reactants and products at equilibrium.
Constructing the Expression for Kc
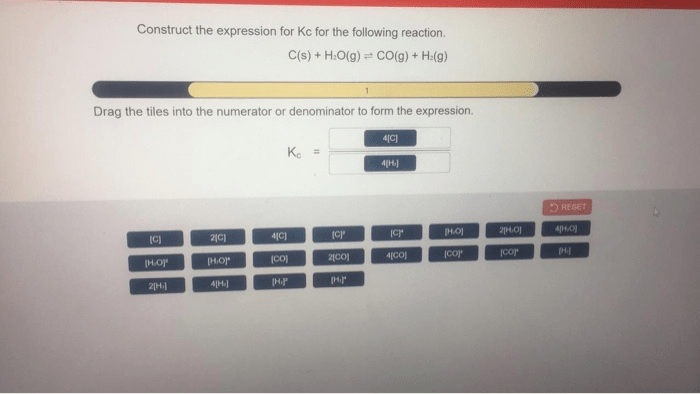
The equilibrium constant (Kc) is a quantitative measure of the extent to which a chemical reaction proceeds towards completion. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium. For a general reaction:
aA + bB ⇌ cC + dD
the equilibrium constant expression is given by:
Kc = [C]^c[D]^d / [A]^a[B]^b
where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species.
Detailed FAQs: Construct The Expression For Kc For The Following Reaction.
What is the significance of Kc in chemical reactions?
Kc provides a quantitative measure of the extent to which a reaction proceeds towards completion, allowing us to predict the reaction’s spontaneity and equilibrium concentrations.
How is the expression for Kc constructed?
The expression for Kc is constructed using the coefficients of the reactants and products in the balanced chemical equation. The coefficients are raised to the power of their respective stoichiometric coefficients.
What are the limitations of using Kc?
Kc assumes ideal behavior of gases and solutions, and it does not account for the effects of temperature or non-ideal conditions.