Embark on a scientific adventure with the Equilibrium and Le Chatelier’s Principle Lab, where you’ll delve into the fascinating world of chemical equilibrium and its dynamic interplay with external factors. This lab promises an engaging exploration of how reactions respond to changes in concentration, temperature, and pressure, shedding light on the fundamental principles that govern chemical systems.
Through a series of carefully designed experiments, you’ll witness firsthand how Le Chatelier’s principle dictates the shift in equilibrium position, providing valuable insights into the behavior of chemical reactions and their applications in various scientific disciplines.
Equilibrium and Le Chatelier’s Principle
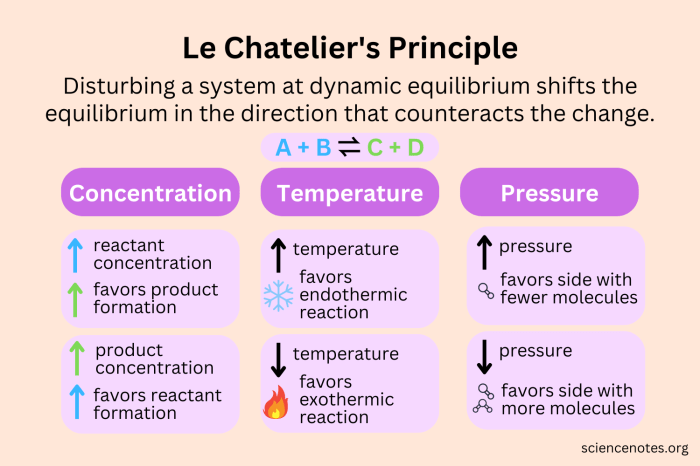
Chemical equilibrium is a state in which the forward and reverse reactions of a reversible reaction occur at equal rates. This means that the concentrations of the reactants and products do not change over time. Equilibrium is achieved when the Gibbs free energy of the system is minimized.
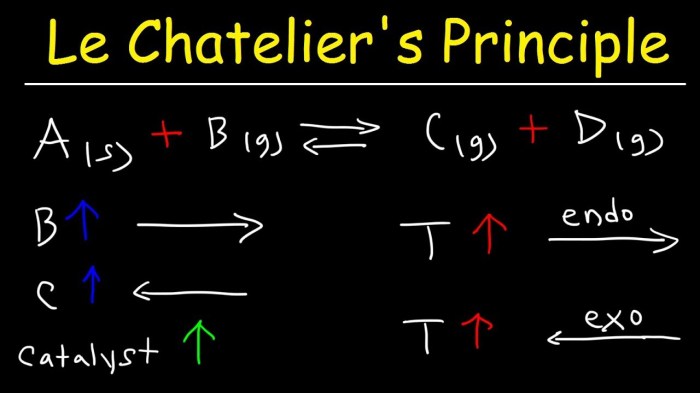
Le Chatelier’s principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that counteracts the change. For example, if the concentration of a reactant is increased, the equilibrium will shift to the product side to reduce the concentration of the reactant.
Experimental Investigation of Le Chatelier’s Principle, Equilibrium and le chatelier’s principle lab
To investigate the effect of changing a specific factor on the equilibrium position of a reaction, an experiment can be designed. The experiment should include the following steps:
- Choose a reaction that is reversible and has a known equilibrium constant.
- Set up the reaction in a closed system.
- Change the value of the factor being investigated (e.g., concentration, temperature, pressure).
- Measure the equilibrium concentrations of the reactants and products.
- Calculate the equilibrium constant for the reaction.
Analysis of Experimental Results
The experimental data can be used to calculate the equilibrium constant for the reaction under different conditions. The equilibrium constant can then be plotted against the varied factor to show the relationship between the equilibrium position and the factor.
The results of the experiment can be discussed in terms of Le Chatelier’s principle. The data should support the theory that the equilibrium position of a reaction will shift in a direction that counteracts the change of condition.
Applications of Le Chatelier’s Principle
Le Chatelier’s principle has many applications in various fields, such as industry, medicine, and environmental science. For example, Le Chatelier’s principle can be used to:
- Optimize the production of chemicals in industry.
- Control the pH of solutions in medicine.
- Predict the behavior of environmental systems.
Understanding equilibrium and Le Chatelier’s principle can aid in optimizing processes and predicting outcomes in a wide variety of applications.
Key Questions Answered: Equilibrium And Le Chatelier’s Principle Lab
What is the concept of chemical equilibrium?
Chemical equilibrium occurs when the forward and reverse reactions in a reversible chemical reaction proceed at the same rate, resulting in no net change in the concentrations of reactants and products.
How does Le Chatelier’s principle predict the shift in equilibrium position?
Le Chatelier’s principle states that if a stress is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. For example, if the concentration of a reactant is increased, the equilibrium will shift towards the product side to consume the added reactant.
What are some practical applications of Le Chatelier’s principle?
Le Chatelier’s principle finds applications in various fields, such as optimizing industrial processes, designing drug delivery systems, and controlling environmental pollution by manipulating equilibrium reactions.