Welcome to our comprehensive grams to moles calculations worksheet, meticulously designed to guide you through the intricacies of converting mass to moles. This worksheet delves into the fundamental concepts of moles, their significance in chemistry, and the intricate relationship between grams and moles.
Embark on a journey of discovery as we unveil the secrets of grams to moles conversions, empowering you with the knowledge to navigate chemical reactions with precision and confidence.
Throughout this worksheet, you will encounter a series of practice problems that will challenge your understanding and hone your problem-solving skills. Step-by-step solutions are provided to illuminate the conversion process, ensuring that you grasp every nuance of this essential chemistry concept.
Dive into the realm of stoichiometry and witness the practical applications of grams to moles calculations, enabling you to determine the precise amounts of reactants and products involved in chemical reactions.
1. Introduction to Grams to Moles Calculations
Moles are a fundamental unit of measurement in chemistry, representing the amount of a substance present. They provide a convenient way to express the quantity of a substance in terms of its atomic or molecular mass. The conversion between grams and moles is essential for various chemical calculations.
2. Methods for Grams to Moles Conversion
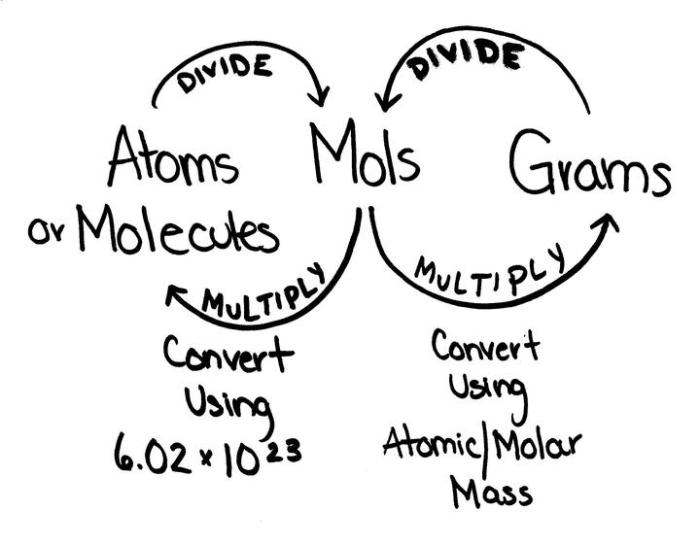
The formula for converting grams to moles is: moles = grams / molar mass.
The molar mass of a substance is its mass per mole, expressed in grams per mole (g/mol). It is crucial to use the correct molar mass for the substance being converted.
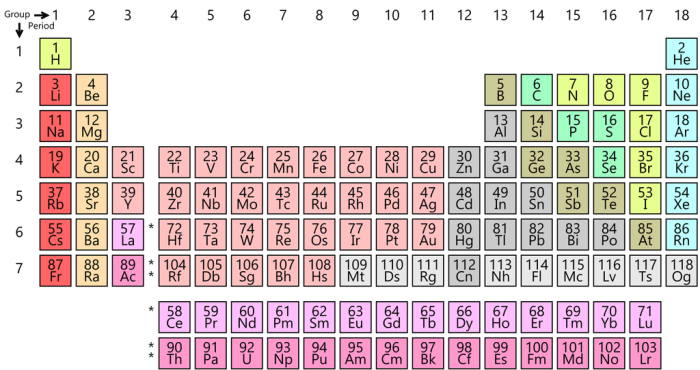
Molar masses can be found using the periodic table. The atomic mass of an element, expressed in grams per mole, is given in each element’s square on the periodic table. For compounds, the molar mass is the sum of the atomic masses of all the constituent atoms.
3. Practice Problems
| Grams | Molar Mass (g/mol) | Moles |
|---|---|---|
| 5.00 g | 18.02 g/mol | 0.277 mol |
| 12.50 g | 32.07 g/mol | 0.389 mol |
| 25.00 g | 63.55 g/mol | 0.393 mol |
Step-by-Step Solution:
- Convert grams to moles using the formula: moles = grams / molar mass.
- For example, to convert 5.00 g of water (H2O) to moles, we use the molar mass of water, which is 18.02 g/mol:
- moles = 5.00 g / 18.02 g/mol = 0.277 mol
4. Applications of Grams to Moles Calculations
Grams to moles conversions are widely used in stoichiometry, which involves calculating the quantitative relationships between reactants and products in chemical reactions.
By converting grams of reactants to moles, we can determine the number of moles of each reactant involved in the reaction. This information allows us to predict the amount of products formed and balance chemical equations.
5. Common Errors and Troubleshooting
Common Errors:
- Using the incorrect molar mass for the substance.
- Not converting grams to moles before performing other calculations.
- Mixing up units (e.g., using grams instead of moles in calculations).
Troubleshooting:
- Double-check the molar mass used for the conversion.
- Ensure that grams are converted to moles before proceeding with further calculations.
- Pay attention to the units used and make sure they are consistent throughout the calculation.
Question & Answer Hub: Grams To Moles Calculations Worksheet
What is the formula for converting grams to moles?
Moles = Grams / Molar Mass
How do I find the molar mass of a substance?
The molar mass is the sum of the atomic masses of all the atoms in the substance’s formula. You can find the atomic masses in the periodic table.
Why is it important to use the correct molar mass in grams to moles calculations?
Using the incorrect molar mass will result in an incorrect number of moles, which can lead to errors in subsequent calculations.