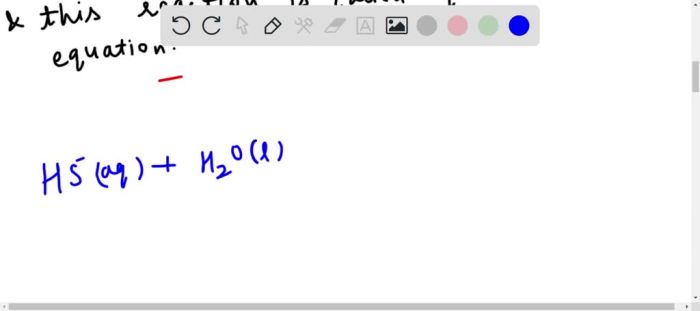
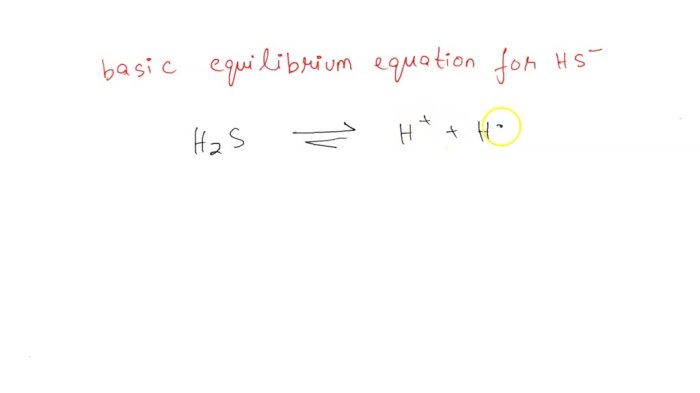
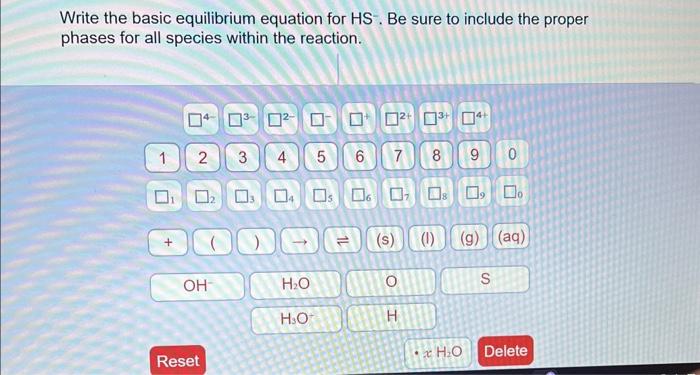
The basic equilibrium equation for HS- unveils the fundamental principles governing chemical reactions and their behavior under varying conditions. This equation, rooted in the law of mass action, provides a mathematical framework for predicting and analyzing the dynamics of chemical systems at equilibrium.
Delving into the realm of equilibrium reactions, we explore the concept of a balanced state where opposing reactions occur at equal rates, resulting in no net change in the concentrations of reactants and products. The equilibrium constant, a pivotal concept in this equation, quantifies the extent to which a reaction proceeds towards completion.
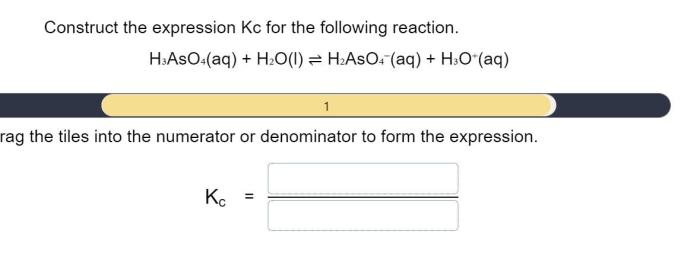
Basic Equilibrium Equation
In chemical reactions, equilibrium is a state where the forward and reverse reactions occur at equal rates, resulting in no net change in the concentrations of the reactants and products. The law of mass action mathematically expresses this concept, stating that the rate of a reaction is directly proportional to the concentrations of the reactants.
The basic equilibrium equation is: $$\textaA+\textbB⇌\textcC+\textdD$$ where A, B, C, and D represent chemical species, and a, b, c, and d are their respective stoichiometric coefficients.
Examples of equilibrium reactions include:
- The Haber process: N 2+ 3H 2⇌ 2NH 3
- The dissociation of water: H 2O ⇌ H ++ OH –
- The formation of carbon dioxide: CO + H 2O ⇌ CO 2+ H 2
Equilibrium Constant
The equilibrium constant (K eq) is a numerical value that represents the extent to which a reaction proceeds towards completion. It is calculated from the concentrations of the reactants and products at equilibrium.
The expression for K eqis: $$K_\texteq=\frac[\textC]^c[\textD]^d[\textA]^a[\textB]^b$$ where [A], [B], [C], and [D] are the equilibrium concentrations of the respective species.
The magnitude of K eqindicates the position of equilibrium:
- K eq> 1: The products are favored at equilibrium.
- K eq< 1: The reactants are favored at equilibrium.
- K eq= 1: The reactants and products are present in equal concentrations at equilibrium.
Factors Affecting Equilibrium
Several factors can affect the position of equilibrium, including:
- Temperature:Increasing temperature shifts the equilibrium towards the endothermic reaction (the one that absorbs heat).
- Pressure:Increasing pressure shifts the equilibrium towards the side with fewer moles of gas.
- Concentration:Increasing the concentration of reactants shifts the equilibrium towards the products, while increasing the concentration of products shifts the equilibrium towards the reactants.
These factors can be used to manipulate equilibrium to obtain desired products.
Applications of Equilibrium Principles
Equilibrium principles have numerous applications in various fields, including:
- Industrial processes:Controlling the equilibrium of reactions in industrial processes to optimize product yield and efficiency.
- Environmental chemistry:Understanding the equilibrium of chemical reactions in the environment to assess their impact on ecosystems.
- Biological systems:Regulating the equilibrium of biochemical reactions to maintain cellular homeostasis and function.
Equilibrium calculations are also used to solve real-world problems, such as determining the solubility of gases in liquids, predicting the outcome of chemical reactions, and designing chemical processes.
FAQ Explained: Basic Equilibrium Equation For Hs-
What is the significance of the equilibrium constant?
The equilibrium constant provides a quantitative measure of the extent to which a reaction proceeds towards completion, indicating the relative amounts of reactants and products at equilibrium.
How can the equilibrium position be shifted?
The equilibrium position can be shifted by altering factors such as temperature, pressure, and concentration, which influence the rates of the forward and reverse reactions.
What are some practical applications of the basic equilibrium equation?
The basic equilibrium equation finds applications in various fields, including industrial processes (e.g., ammonia production), environmental chemistry (e.g., acid-base reactions in water bodies), and biological systems (e.g., enzyme-catalyzed reactions).